How To Work Out Relative Atomic Mass
To work out the relative atomic mass of an element all you need to do is multiply each isotopic mass by its relative abundance add all the values together and. As a result relative atomic mass takes into account all of the naturally occurring stable isotopes of an element.
Solved 4 Why Are Relative Atomic Masses Used And Why Is C Chegg Com
To calculate formula mass multiply the subscript of each element in the formula by the elements atomic weight relative atomic mass found on the periodic table.
. Average mass relative atomic mass of copper 63 x 692 65 x 308 100 636-Example 14. The formula for relative atomic mass is isotope mass x isotope abundance 100. The relative atomic mass of an element is a weighted average of the masses of the atoms of the isotopes because if there is much more of one isotope then that will influence the average.
The mass numbers of its isotopes the abundance of these isotopes Chlorine Chlorine naturally exists as two isotopes _. Given that the percentage abundance of is 75 and that of is 25 calculate the A r of. The relative atomic mass of an element is a weighted mean mass of the isotopes of an element compared with that of the 12 C isotope.
Relative Atomic Mass Ar 2 X 185 3 X 187 2 3 186 3 sig. The answer is the total atomic mass or atomic weight of the element. To work out the relative atomic mass of an element all you need to do is multiply each isotopic mass by its relative abundance add all the values together.
The formula for relative atomic mass is A r average mass of isotopes of the element Example. The strict definition of relative atomic mass A r is that it equals the average mass of all the isotopic atoms present in the element compared to 1 12 th the mass of a carbon-12 atom. 465 70 votes.
The average mass is the relative atomic. What is the relative. Silver atoms consist of 514 of the isotope 107 Ag and 486 of the isotope 109.
Calculate the relative atomic mass of rhenium to three significant figures. The relative atomic mass Ar of an element is calculated from. The weighted mean mass of chlorine is 355.
Calculating Relative Atomic Mass from Mass Spectra - YouTube 000 618 Calculating Relative Atomic Mass from Mass Spectra 92738 views Nov 22 2015 A look at. In order to perform accurate chemical calculations relative atomic mass must be used rather than an individual mass number. How To Calculate Relative Atomic Mass Chemical Calculations Chemistry FuseSchool - YouTube In this video we are going to learn how to calculate the relative.
Relative molecular mass Mr is the weighted average of the mass of a molecule compared to 112 of. You are given a sample containing 98 carbon-12 and 2 carbon-13. The unit for formula mass.

Toppr Ask Question
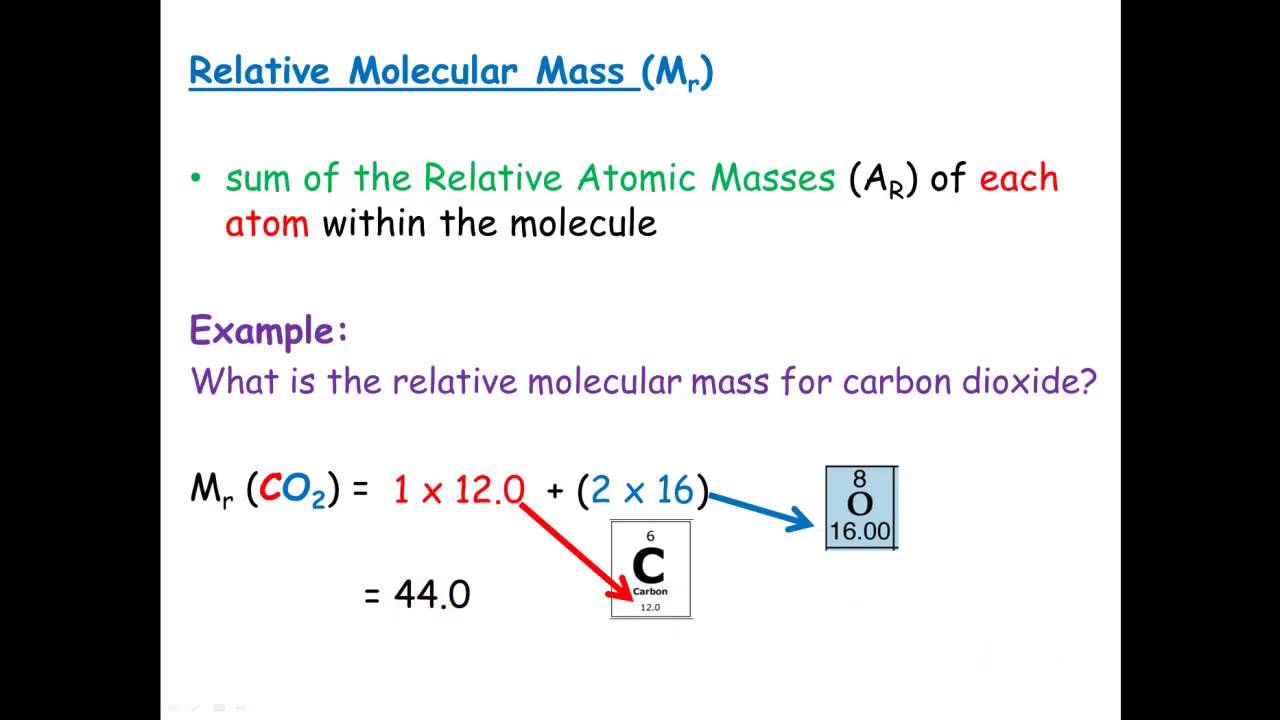
Relative Molecular Mass Relative Formula Mass Youtube

Chlorine Has Two Isotopes Of Atomic Mass Units 34 97 And 36 97 The Relative Abundance Of An Isotope Is 0 755 And 0 245 Respectively Find The Average Atomic Mass Of Chlorine

Calculate Relative Mass 1 5 2 Edexcel Igcse Chemistry Revision Notes 2019 Save My Exams
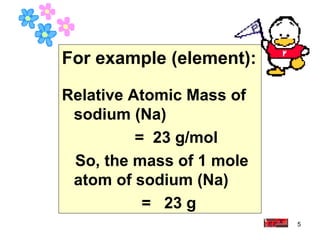
3 3 B Relative Atomic Mass
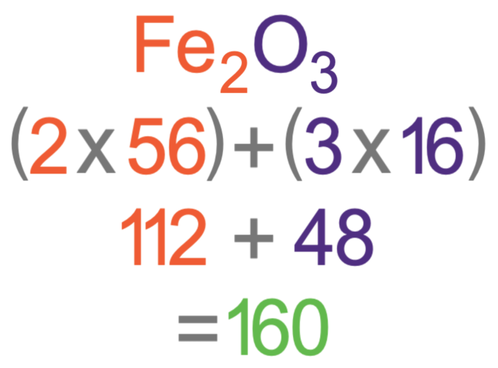
C3 A Relative Formula Mass Aqa Combined Science Trilogy Elevise

Atomic Mass Calculations

Definition Of Isotopes And Relative Atomic Mass Solutions

Calculating The Relative Atomic Mass Of Lithium Gcse Science Marked By Teachers Com

Determination Of The Relative Atomic Mass Of Lithium Gcse Science Marked By Teachers Com

The Relative Atomic Mass Of An Element Is 10 28
How Can We Explain Relative Atomic Mass Quora

A Calculate The Relative Molecular Mass Of Water B Molecular Mass
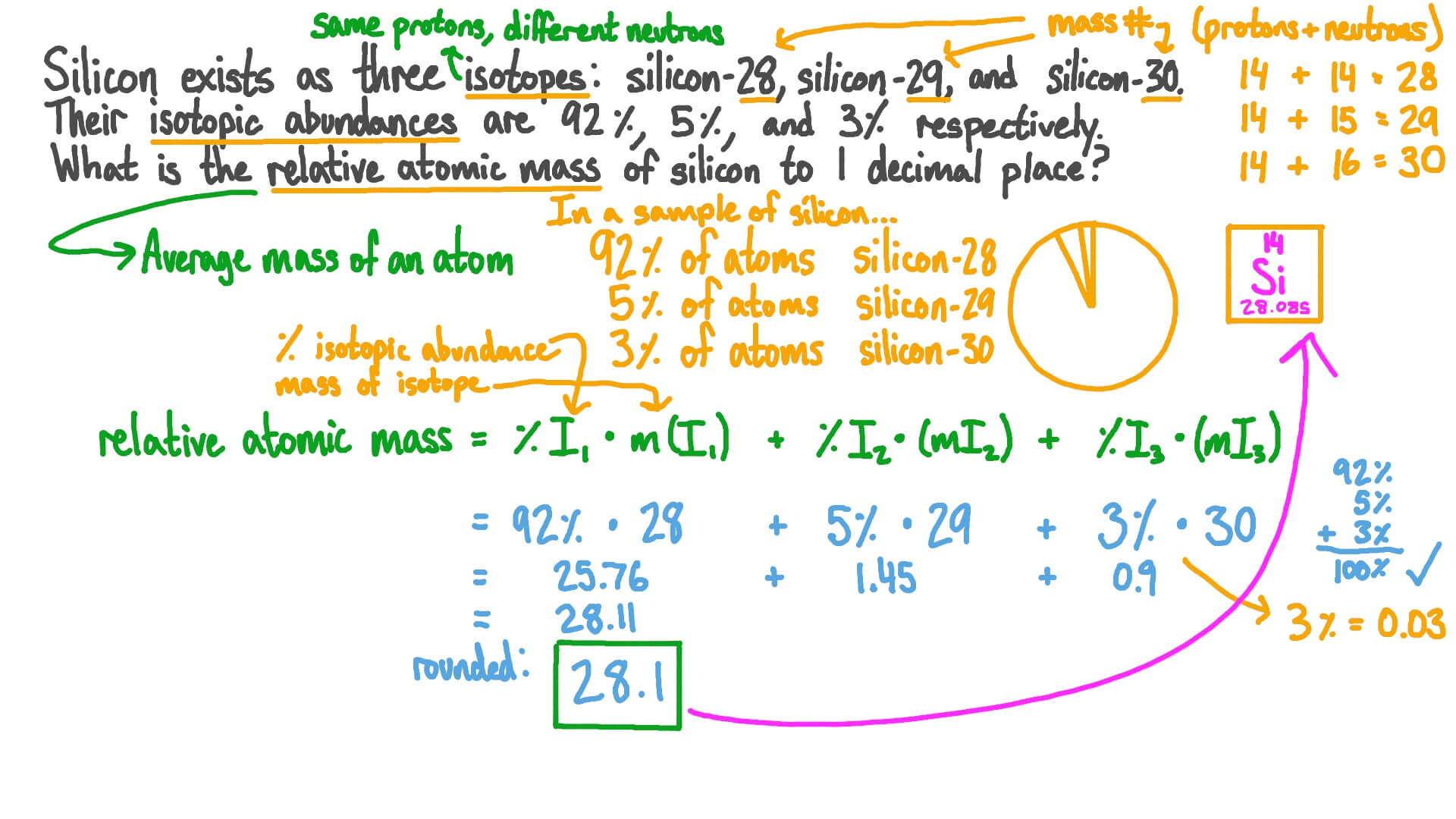
Question Video Using Relative Abundance Of Isotopes To Calculate Relative Atomic Mass Nagwa
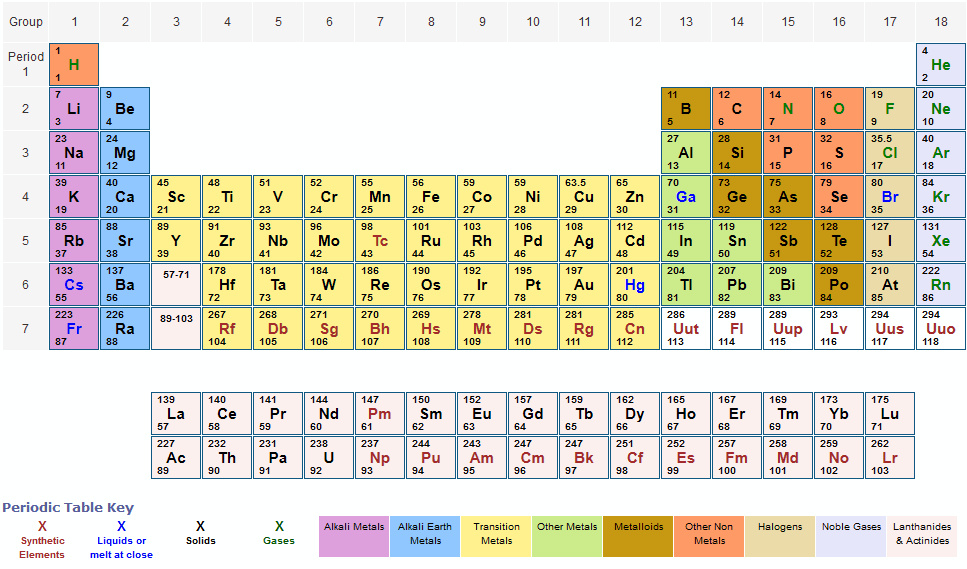
Periodic Table Of Elements With Relative Atomic Masses

Relative Formula Mass Worksheet Edexcel Gcse Science

Relative Atomic Mass And Moles Sort Into Order Worksheet Quickworksheets